We are the leading transatlantic small molecule contract research and manufacturing organization that has been a trusted global partner for biotech and pharma companies for over 30 years.
About us
Why work with us?

Vacancies
A career at Symeres is unlike any other. Groundbreaking research, inspiring clients, motivating projects and being part of talented teams. So if you’re an outstanding talent with a need to discover the solutions to all kinds of fascinating challenges, we want to hear from you.

Latest news
Below are a few of our most recent news items.
-

Organix: New facility
On March 26th we had the Grand Opening of our brand new, state-of-the-art Organix synthetic and analytical chemistry laboratories in Woburn, just outside Boston MA.
Read more
-
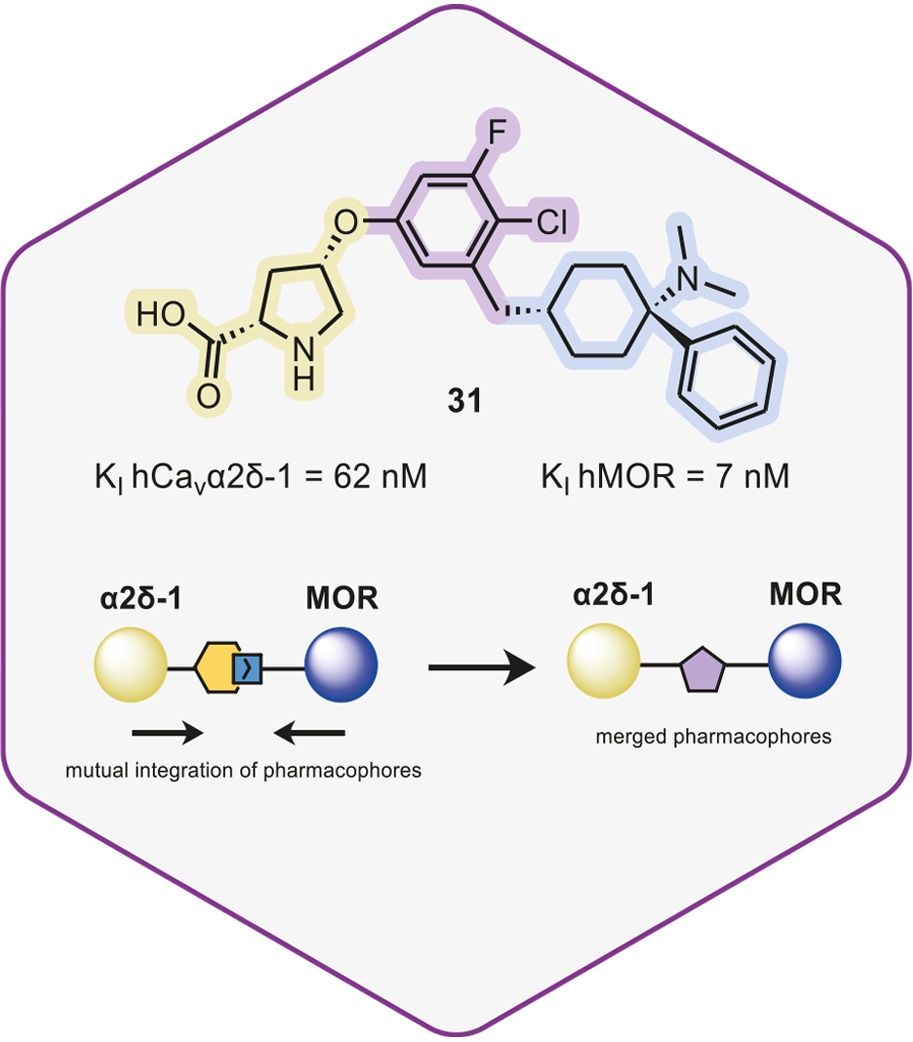
Towards Multitargeted Ligands as Pain Therapeutics: Dual Ligands of the Cavα2δ-1 Subunit of Voltage-Gated Calcium Channel and the μ-Opioid Receptor
In collaboration with WeLab Barcelona, we report the synthesis and biological activity of novel pain therapeutics, which target both Cavα2δ-1 and MOR. One of these disubstituted pyrrolidines demonstrated an analgesic effect in various rodent models of pain, and a lack of unfavorable off-target interactions, showing no evident signs of withdrawal or tolerance. Click on the link below for a short summary of this publication.
Read more
-
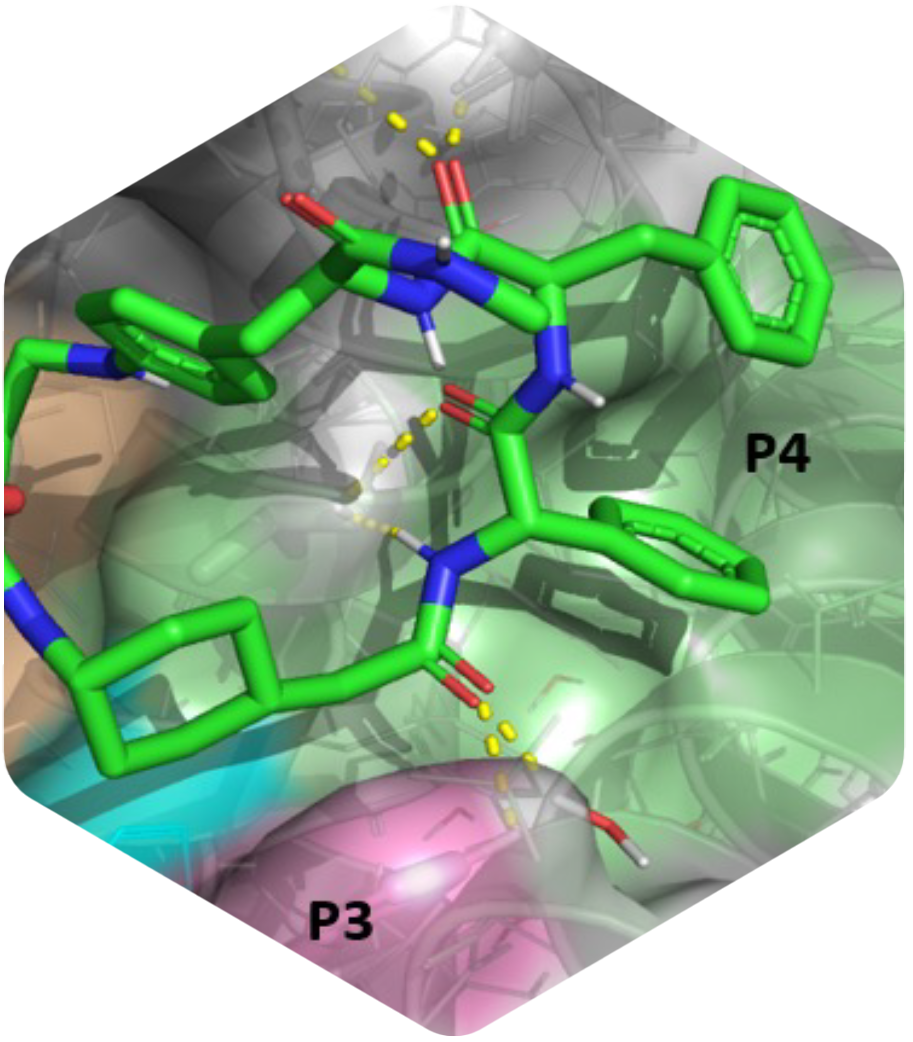
Innovative macrocycles as powerful Mcl-1 inhibitors
This is a recap of the article “Development of potent Mcl-1 inhibitors: structural investigations on macrocycles originating from a DNA-encoded chemical library screen” published in the Journal of Medicinal Chemistry. Myeloid cell leukemia 1 (Mcl-1) is a protein that plays a crucial role in the regulation of cell survival and cell death. It is part of a family of proteins called the Bcl-2 family, which are known for their involvement in controlling the life span of cells.
Read more
-

Oncolines unveils new State-of-the-Art Laboratories
In a significant stride towards advancing cancer research and drug development, Oncolines, has officially received the keys to its new laboratories and offices situated within the prestigious Curie Building on the Pivot Park in Oss, the Netherlands.
Read more
-

Our Article Published in the Journal of Medicinal Chemistry
Symeres is thrilled to announce the publication of a scientific article in the prestigious Journal of Medicinal Chemistry with our colleague Koen Hekking as the main author and many more Symerians involved! We are incredibly proud of our colleagues who were instrumental in the research and development of this innovative project.
Read more
-

Peter Molenveld Assumes Role as Secretary of KNCV (Royal Netherlands Chemical Society)
Symeres is proud to announce that our colleague Dr. Peter Molenveld has been appointed as a board member of the Royal Netherlands Chemical Society (KNCV). His appointment as Secretary marks a crucial milestone in Molenveld’s career and signifies his commitment to advancing the interests of the chemical community in the Netherlands.
Read more
-

Luc van Hijfte Assumes Presidency of EFMC, Spearheading Advances in Medicinal Chemistry and Chemical Biology
Symeres is thrilled to announce that as of January 1, 2024, our sr. Vice President Dr. Luc van Hijfte has taken the helm as the President of the European Federation of Medicinal Chemistry and Chemical Biology (EFMC).
Read more
-
Interview with the Computer-Aided Drug Design (CADD) Department
Our Computer Aided Drug Design department supports our clients’ drug discovery projects with some of the best (predictive) software.
Read more
-

“If the compound can be made, Symeres will undoubtedly find out a good way to make it.” Dooyoung Jung, CEO of Pinotbio.
An in-depth interview with Doo Young Jung, the founder and CEO of our client Pinotbio in South Korea, focused on the challenge of cancer resistance/relapse
Read more
-

New video of our Solid-State Center of Excellence
In this new video our colleagues Gabriella Pizutti, Chantalle van Berkel, and Eva Verhofstad, will give you an exciting lab tour through the Symeres Solid-State Centre of Excellence, located at the Symeres site in Weert, the Netherlands. You will learn more about PhysChem Properties, Solid Form Screening, Crystallization, Crystal Habit, Pre-formulation, and Formulation. Feel free to contact us via the contact form on this website if you would like to learn more about our Solid-State capabilities.
Read more
-
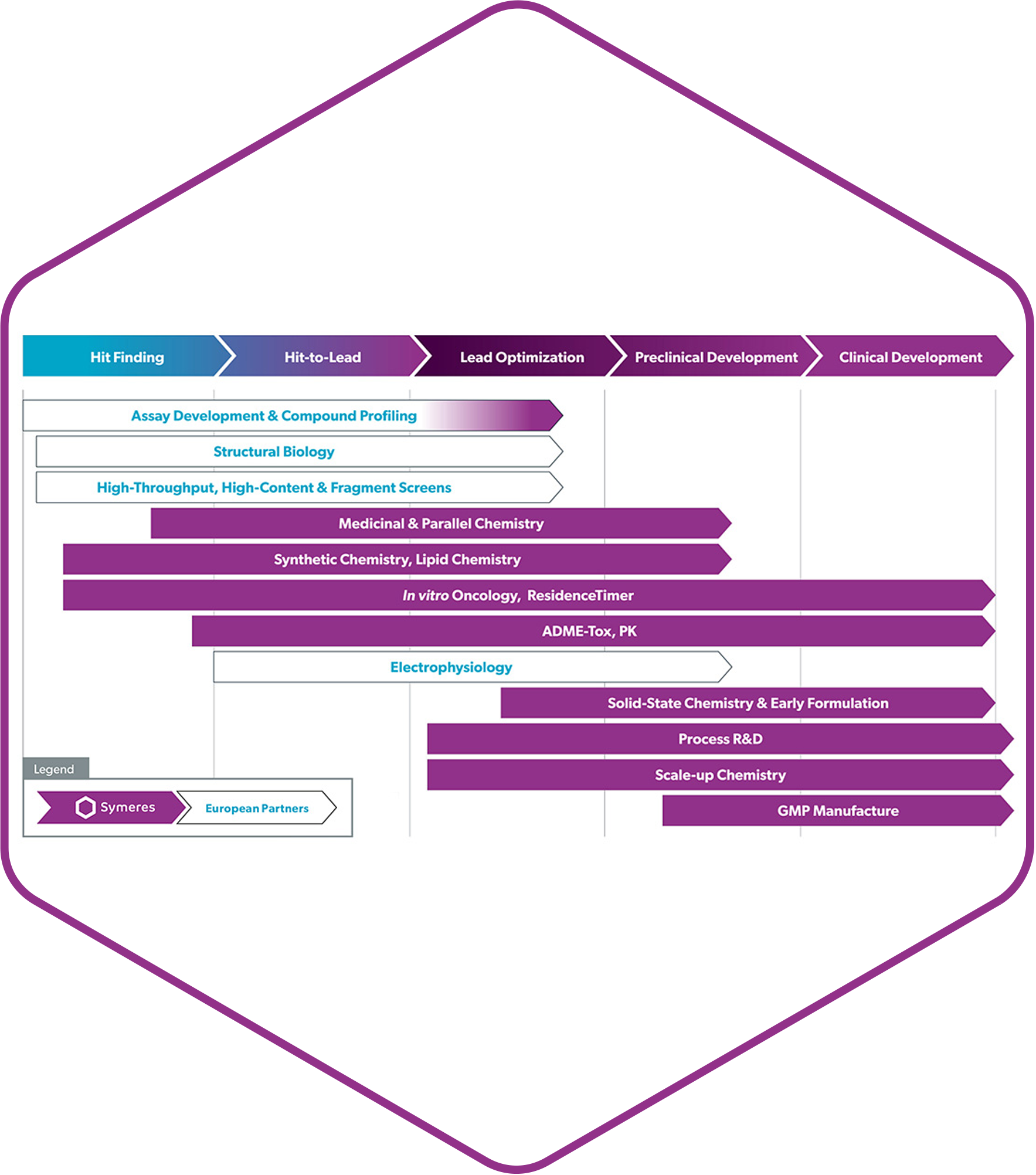
New scientific services from Symeres
We’re proud to announce that our brand new CMC facility at the Exemplify site of Symeres in New Jersey has been successfully completed. GMP drug product R&D facilities that will allow it to provide end-to-end services ranging from GMP and non-GMP drug product development to phase 1 and 2 clinical supplies of various oral, parenteral, and topical formulation dosage forms.
Read more
-

Interview with the new Managing Director of Symeres Groningen
On October 2, Dr Melloney Dröge started in her new role as Managing Director for the Groningen site.
Read more
-
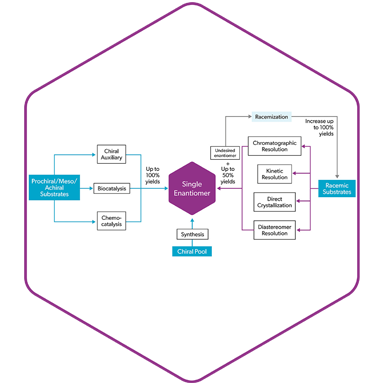
Chiral Chemistry: in the DNA of Symeres
Over 50% of current drugs in development are chiral. At Symeres we apply a suite of state-of-the-art technologies to obtain the desired chiral compound in high enantiomeric purity. For decades, Symeres has been supporting the global pharma and biotech industry with the synthesis and delivery of chiral compounds, both through synthesis and purification strategies.
Read more
Latest events
Symeres actively participates in many international Life Sciences conferences. See below for some of the upcoming events where you can meet us and discuss your R&D services needs.
-

EFMC-ACSMEDI Medicinal Chemistry Frontiers 2024
8-11 April
Utrecht, The NetherlandsRead more
-

40th SCI Process Development Symposium
10-12 April
Cambridge, UKRead more
-

CPHI Japan 2024
17-19 April
Tokyo, JapanRead more
-

Swiss Biotech Day 2024
22-23 April
Basel, SwitzerlandRead more
-

35th Symposium on Medicinal Chemistry in Eastern England
25 April
Stevenage, UKRead more
Feel free to contact us!
Curious to know more about what can we do for you? Get in touch and let’s start a conversation.
