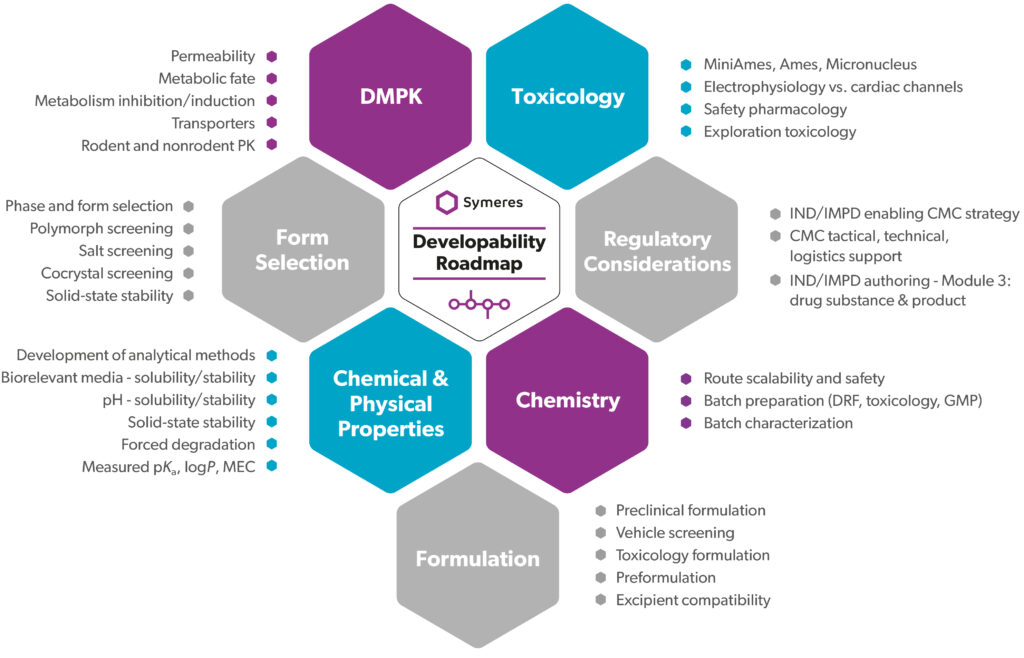
Drug discovery and development entail a multifaceted journey, from identification to market approval. Our proprietary Developability Roadmap package streamlines this process, providing a clear plan of evaluation and action for transitioning from discovery to development.
Comprising data from various technical disciplines, including synthetic chemistry, materials science, formulation, ADME, and toxicology, our roadmap ensures comprehensive assessment and mitigation of potential issues or delays. With most studies being conducted in house and selected aspects being overseen by trusted third parties, we deliver a single, meticulously compiled document to facilitate your IND/IMPD filing.
We provide insights backed up with data. Symeres collaborates with your team to identify risks, propose mitigation strategies, and coordinate future activities. By entrusting us with your Developability Roadmap, you save invaluable time and benefit from our industry experience and efficient project management.
Discover Exemplify
We deliver a number of services in partnership with Exemplify BioPharma, a global Partnership Research Organization (PRO) offering end-to-end CMC solutions under one roof.
Exemplify offers:
- Integrated project management across Process Chemistry, Analytical Chemistry, and Formulation Development
- Expert oversight on CRO/CMO selection, contracting, and management
- Comprehensive support with CMC regulatory submissions (IND, IMPD, NDA, MAA)
- Full Quality Assurance and compliance sign-off
- Efficient timeline and budget management
Their newly expanded Formulation Suite also opens the door to enhanced formulation capabilities, strengthening support from early development through to commercialization.
