Design and synthesis from hit to clinical candidate

Drug discovery is an interdisciplinary science where experience, communication, innovation and speed are key. An experienced interdisciplinary team will work with you on your next clinical candidate.
Symeres delivers high-quality integrated medicinal chemistry services to advance your drug discovery programs: from hit identification to lead optimization and nomination of a clinical candidate. Our team combines highly experienced leadership and project scientists with extensive resources, such as parallel synthesis and purification, supported by our computational chemistry and our Admescope in vitro ADME-Tox and PK platform. Additionally we can integrate a range of in vitro biology capabilities, including Oncolines and ResidenceTimer.
We also have strategic partnerships with carefully selected European CROs for specific biology and structural biology platforms, offering an integrated solution to your Drug Discovery services needs.
This combination of expertise and infrastructure allows the rapid multiparameter expansion and optimization of compound series.
Hit Generation
Symeres has a strong track record in the validation and expansion of hits derived from different screening platforms, including high-throughput screens (HTS), fragment screens, DNA-encoded libraries, and AI-based virtual screens.
A cornerstone of our hit-finding activities is our 75,000 compound Symegold library, created as part of the European Lead Factory (ELF) IMI-2 consortium. The compounds in this collection were based on diverse lead-like properties and structural novelty.
The Symegold library can be accessed at the Axxam screening centers and screened alongside a series of other high-quality libraries assembled and curated by Axxam.
We have significant expertise in validating and prioritizing HTS hits, taking into account principles of lead-likeness, synthetic feasibility, IP, and ADME properties. Our parallel chemistry platform can play a decisive role in rapid hit expansion, developing both structure–activity and structure–property relationships for hit series. This, coupled with a comprehensive ADME-Tox platform, allows project teams to make informed decisions based on a solid data package.
Hit-to-Lead
Starting on the right foot is essential in drug discovery, and the correct choice of hit classes to take forward will influence the entire project downstream.
Hits can be generated from multiple sources. Our experienced medchem teams evaluate hits from fragment-based approaches; medium- and high-throughput screens including those exploiting our innovative 75,000 compound Symegold library; as well as those based on literature starting points.
The initial evaluation can entail building the most effective screening cascades to assess both activities and potential liabilities, including appropriate application of our in vitro ADME profiling platform for the phase of the project, as well as involving our computational chemistry capabilities for in silico property calculations and building hypotheses. Wherever appropriate, we apply our parallel synthesis and purification platform to efficiently map structure–activity and structure–property relationships and help evaluate which are the most promising hit classes to progress.
Rapid evaluation of hit classes enables the generation of initial structure–activity and structure–property relationships, alongside an initial IP evaluation and target-candidate profile generation, to help de-risk subsequent lead-optimization efforts in a time- and cost-effective manner.
Lead Optimization
Lead optimization requires fine-tuning multiple parameters. Symeres has an excellent track record of progressing programs through to candidate selection.
Clients often approach us with chemical starting points requiring optimization of a single or multiple parameters, such as solubility, hERG, CYP inhibition etc.
Our in-house SAR database solutions, coupled to DataWarrior and Schrödinger’s LiveDesign interfaces, allow us to effectively mine and analyze project data to efficiently design analogues and progress your lead series toward candidate selection. In many cases we integrate our parallel synthesis and purification infrastructure, generating arrays of analogs to rapidly build structure-activity and structure-property relationships, which we use to design the next generations of ligands.
Computational Chemistry
From bioisosteric replacements to interactive structure and ligand-based drug design, computational chemistry is an integral part of many of our medicinal chemistry projects. Our computational chemists have a solid background in synthetic chemistry and factor synthetic feasibility into their molecular design activities.
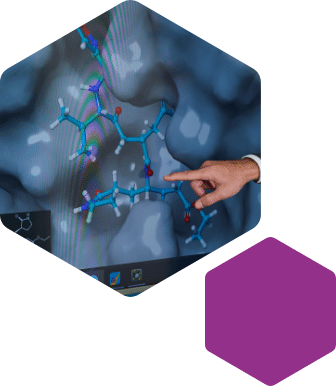
At Symeres, we develop modeling approaches tailored to customer requirements. Our arsenal of molecular modeling software includes Spark, MOE, and Maestro, and we employ state-of-the-art protein and data visualization software to generate insight into how lead molecules bind to the target protein, either via experimental data or molecular docking.
Our Medicinal Chemistry teams employ advanced data visualization and analysis tools, such as DataWarrior and LiveDesign, which allow for expert property interrogation, trend studies, and R-group analyses. Our workflows allow our chemists to submit design ideas for automatic docking to filter and refine their design hypotheses.
We offer molecular modelling as an integrated component of any medicinal chemistry project and as a stand-alone service. To learn more about the approach that we take to computational medicinal chemistry, click here to read an interview with our team.
Rapid Analog Synthesis
Symeres has a dedicated team for the rapid design, synthesis, and purification of compound arrays.

Parallel array synthesis is a powerful tool to rapidly explore the chemical space around a hit compound. At Symeres, we have developed streamlined workflows to optimize our library design, synthesis, and purification. The equipment our chemists use is semiautomated, allowing both a speedy process and room for customization. We have multiple platforms available to adapt the setup of the library to your specific needs, whether this is a few compounds or a few hundred. Our platform is also adaptable to larger-sized array synthesis over longer timescales, and this was used to design and generate our Symegold library of 75,000 lead-like compounds, as part of the European Lead Factory initiative. The Symegold library is available for screening at our strategic partner, Axxam.
Integrated Drug Discovery
Symeres provides clients looking for integrated drug discovery services with access to a trusted network of high-quality European providers under a single simple contractual agreement.
Symeres has a strong European network of centers of excellence to provide integrated drug discovery for our clients.
Central to this is a strategic alliance with Axxam, a world-class provider of in vitro assay development, screening, and compound profiling services based in Milan, Italy. The combined platforms of Symeres, our wholly owned affiliate Admescope, and Axxam provide the core upon which to build gene-to-candidate discovery programs. Program-specific oncology solutions can also be provided through our Oncolines platform, including cancer cell line proliferation assays, residence time determination, combination studies and mechanistic studies. Additionally, partnerships with world-class companies, such as LeadXPro and Proteros, in areas including structural biology & fragment-based screening through biophysics.
A single contract with Symeres allows our clients access to our network, working together as a single project team.