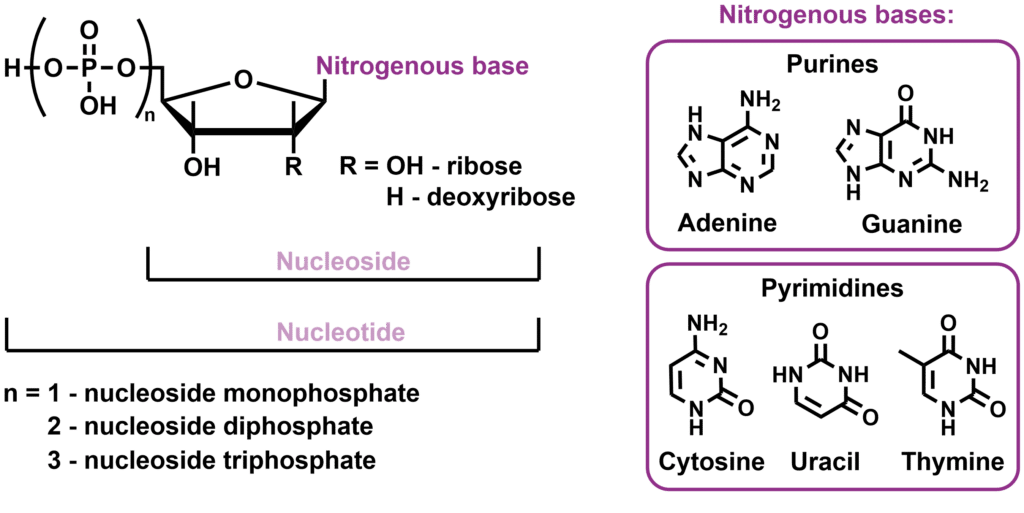
Nucleosides and nucleotides are the monomeric components of nucleic acids, the class of biopolymers that includes DNA and RNA. They play crucial roles in various cellular processes, ranging from storage and reproduction of genetic information to energy transfer and cellular communication, whereas other derivatives serve as cofactors for a range of diverse biocatalytic processes. Nucleosides are composed of a combination of a nitrogenous base (purines or pyrimidines) bound via a glycosidic linkage to a pentose sugar. If the sugar is modified with one or more phosphate ester group, it is called a nucleotide. In nucleic acids, the carbohydrate moieties of the monomers are linked via 3’,5’-phosphate diesters to provide a biopolymer with a linear backbone. This way, the nucleobase moieties are placed in sequential order, which is the molecular basis for the encoding of genetic information.
The nitrogenous bases have unique hydrogen-bonding properties, which make them highly specific to bind in fixed pairs. The cooperative binding of the base pairs forms the basis of the renowned tertiary-helix structure that nucleic acids adapt.
Genetic Information: Nucleotides are the units that make up DNA and RNA, and they carry the genetic information that determines the characteristics and functions of living organisms. The role of DNA is to store genetic information, which encodes the instructions for building and maintaining an organism. It is passed on from one generation to the next and, consequently, requires high stability. For that reason, it uses deoxyribosides for the carbohydrate backbone. RNA plays a more temporary role in gene expression and protein synthesis, and for that reason, it uses ribosides as the carbohydrate backbone, which gives it a lower stability.
Energy Carriers: Nucleotides, such as adenosine triphosphate (ATP) and guanosine triphosphate (GTP), serve as high-energy molecules that power various cellular processes. They store and transfer energy within the cell and are crucial for metabolic reactions. They share the same common unit as some of the building blocks of DNA, but the degree of phosphorylation determines their energetic state. Kinase enzymes catalyze the transfer of high-energy phosphate carriers to specific substrates. This is a way for cells to transmit signals and regulate processes.
Cell Signaling: Certain specific nucleotides and nucleosides, like cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP), are involved in cell-signaling pathways. They transmit signals from the cell surface to the interior, regulating various cellular responses.
Enzyme Cofactors: Nucleotides can serve as cofactors for enzymes, facilitating their catalytic activities. For example, nicotinamide adenine dinucleotide (NAD+) and flavin adenine dinucleotide (FAD) are coenzymes that play essential roles in redox reactions. Both contain the adenosine phosphate moiety.
Nucleosides and nucleotides are generally divided into two main classes: purines, which include adenine and guanine as the bases, and pyrimidines, which include cytosine, uracil, and thymine as the bases. These unique compounds and their interactions serve as the foundation of DNA and RNA molecules.[1] The structures and differences between nucleosides and nucleotides are delineated in Figure 1.
Synthesis of Nucleosides and Nucleotides
Symeres has a strong track record in the challenging synthesis and purification of nucleosides, nucleotides, and closely related analogs. Properties such as high water solubility caused by the presence of polar and charged functional groups, the potential instability of the pyrophosphate bonds, and complex and time-consuming purifications make nucleoside and nucleotide chemistry a specialized field that requires significant hands-on experience, despite the substantial amount of research published in the area.[2],[3] Methods for the efficient preparation of nucleosides and nucleotides have been extensively explored. In the chemical synthesis of nucleic acids, two different approaches can be considered: solution-phase or solid-support synthesis. Both protected and unprotected starting materials can be employed, depending on the types of reagents chosen. Purity determination and purification of nucleosides and nucleotides also bring several challenges. We apply HPLC methods with specific dedicated columns for polar compounds to monitor reaction progress and to determine final purities. Purification methods include preparative HPLC, reverse-phase chromatography, ion-exchange chromatography, or chromatography that uses polystyrene or Sephadex resins.
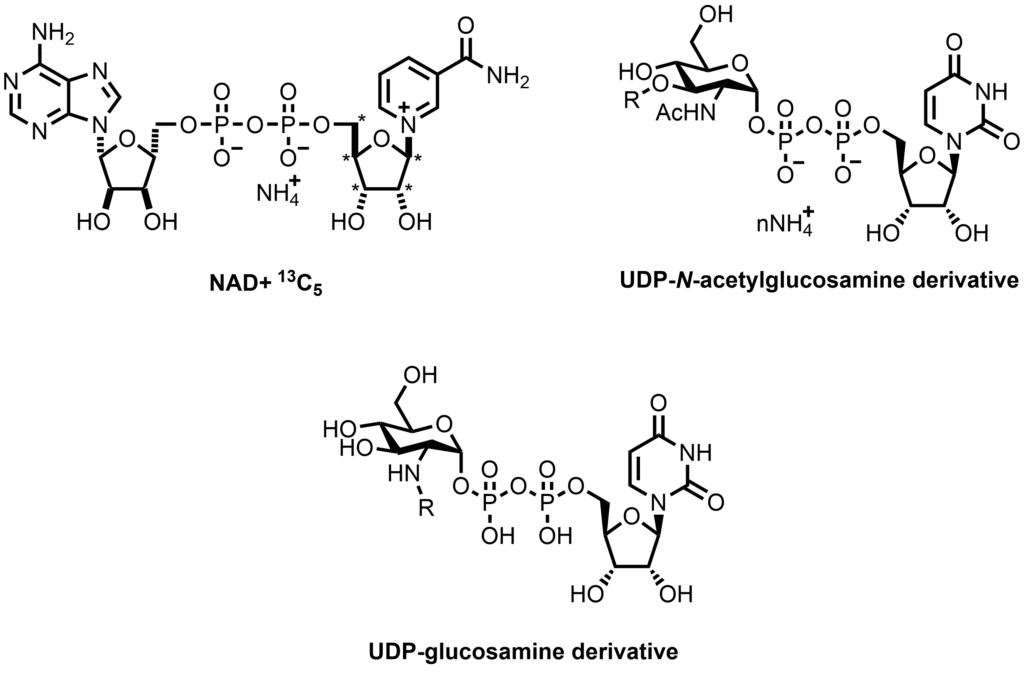
Stable labeled, methylated, or fluorinated analogs; cyclic dimers; and C-linked or other unnatural nucleotides are amongst the analogs that can be synthesized on request. Closely related biologically relevant compounds that share the nucleotide core include coenzyme A (involved in fatty acid synthesis), NAD (a coenzyme central to metabolism), and flavin adenine dinucleotide (FAD, a redox-active coenzyme).
Are you interested in our capabilities around nucleosides and nucleotides? Contact one of our experts directly via the form below.
[1] L. W. Jansonet al., The Big Picture: Medical Biochemistry, Chapter 4: Nucleosides, Nucleotides, DNA, and RNA, 2018
[2] B. Roy et al., Chem. Rev. 2016, 14 , 7854–7897 DOI: https://doi.org/10.1021/acs.chemrev.6b00174
[3] G.K. Wagner et al., Nat. Prod. Rep. 2009, 26 , 1172–1194 DOI: https://doi.org/10.1039/B909621N
