Home / News
Our news

What are the top drug development trends for 2026? Exemplify CSO Paul O’Shea discusses what to watch
Exemplify BioPharma CSO Paul O’Shea shares his perspective on the key drug development trends for 2026, including AI-driven decision-making and drug repurposing strategies.

17.12.25
Symeres earns first SBTi approval as part of long-term sustainability journey
Nijmegen, Netherlands – 17.12.25 – Symeres, a leading contract research, development, and manufacturing organisation (CRDMO), has received its first approval from the Science Based Targets initiative (SBTi) for its greenhouse gas (GHG) emissions reduction targets. This marks a major milestone in Symeres’ environmental strategy and affirms its commitment to building a more sustainable, low-carbon future. […]

01.10.25
Symeres appoints Gabriella Gentile as Chief Operating Officer to drive transatlantic growth
With more than 25 years of international experience in the pharmaceutical, medical device, and services sectors, Gabriella brings a proven track record in operational performance, lean methodologies, and global transformation programs.

10.09.25
Symeres announces acquisition of DGr Pharma
DGr Pharma specializes in preclinical and clinical regulatory strategy and consultancy for biotech and pharmaceutical partners working in early drug development. Its core services include chemical-pharmaceutical, non-clinical and clinical development planning, quality assurance, and regulatory submissions. DGr Pharma has deep expertise in small and large molecules including antibodies, ADCs, and oligonucleotides.

03.06.25
Symeres and Yoneda Labs use AI to optimize cross-coupling reactions
San Francisco, May 29, 2025 – Yoneda Labs, a leader in computational tools for reaction optimization, has successfully collaborated with Symeres, a leading transatlantic, small molecule contract research and manufacturing organization. The aim was to optimize transition metal catalyzed cross-coupling reactions to improve reaction yield and efficiency utilizing Yoneda Labs’ cutting-edge software.

16.12.24
New production lines D1 and D2 at our Prague site
The new production lines D1 and D2 were launched for the first time in May 2024 at the Prague site. The first batch of 11 kg of API was produced in the following weeks, and the tremendous efforts of the many people involved in planning the entire production line have finally paid off. Since the production line was launched, all equipment has been continuously utilized.

06.12.24
New NMR technology – meet “Maggie”
Nuclear Magnetic Resonance (NMR) Spectroscopy is a powerful analytical tool well known to all organic chemists and to anyone that works in pharmaceuticals. By placing a sample dissolved in deuterated solvent into a strong magnetic field (roughly 4000x the strength of Earth’s magnetic field) and pulsing it with radio frequencies, the chemist is given an enormous amount of information from the identity and placement of functional groups to relative stereochemistry of diastereomers. Recently, Exemplify added NMR to our expanding list of capabilities.
05.12.24
Symeres announces leadership evolution
Symeres announces the evolution of its leadership team ahead of the company’s next phase of development. After nearly three decades at the helm, Symeres’ co-founders, Dr. Eelco Ebbers (CEO) and Dr. Frank Leemhuis (Head of Corporate Development), have decided to step back from their current executive roles.

20.09.24
Official opening of new Oncolines home, the Marie Curie Building at the Pivot Park in Oss, the Netherlands
After months of dedication and collaboration, the official opening of the Marie Curie building at the Pivot Park in Oss was opened on Sep 19th, 2024. The Marie Curie building is a multitenant laboratory building which houses Oncolines B.V., the in vitro biology services laboratory of Symeres. Read more about this exciting development!

01.05.24
New equipment at Exemplify
Exemplify has recently acquired a Mettler-Toledo EasyMax 402 automated reactor system to add to its process development capabilities. This instrument can accommodate reactor sizes of 100 and 400 mL and allows for the precise automated control of reaction parameters such as temperature, stirring speed, reagent addition rate, and pH, providing real-time data collection that can greatly enhance our understanding of a reaction’s unique profile.
01.05.24
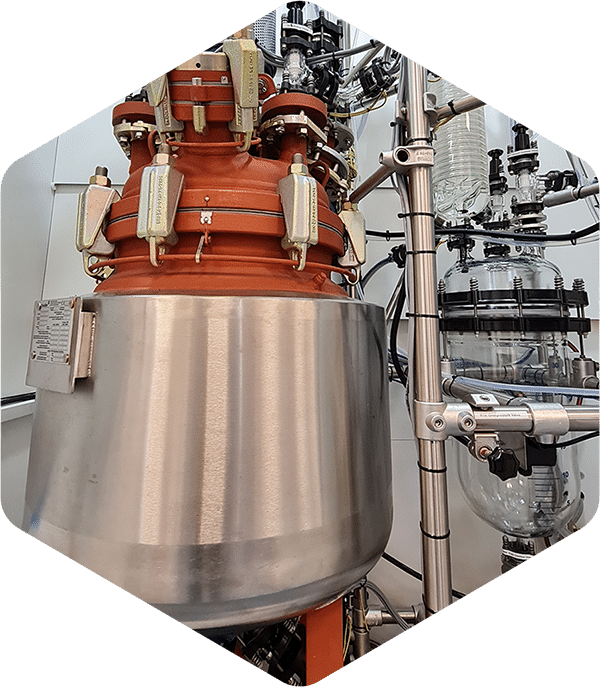
New 100 L vessels in Groningen
The first of the two new 100 L glass-lined stainless steel reactor lines has successfully finished the testing and now it is operational at our Groningen site. By doubling our batch size capacity, we have increased the efficiency for large-scale non-GMP production projects. This will result in faster delivery times for our clients and less batch routine work for our chemists.


