We are delighted to announce the acquisition of DGr Pharma, an internationally operating consultancy and contract research organization (CRO) headquartered in Breda, the Netherlands.
DGr Pharma specializes in preclinical and clinical regulatory strategy and consultancy for biotech and pharmaceutical partners working in early drug development. Its core services include chemical-pharmaceutical, non-clinical and clinical development planning, quality assurance, and regulatory submissions. DGr Pharma has deep expertise in small and large molecules including antibodies, ADCs, and oligonucleotides.
Their broad service offering includes:
- Project Management: coordinating everything from individual trials to full development programs.
- Non-clinical Development: planning and managing non-clinical studies to support progression into the clinic.
- Clinical Development – Consultancy: providing strategic and operational guidance for clinical studies.
- Pharmacokinetics – Data Analysis: delivering expert analysis of drug concentration–time data across all development stages.
- CMC Services: ensuring robust Chemistry, Manufacturing and Control (CMC) to support development and regulatory submissions.
- Regulatory Affairs: navigating global requirements with tailored regulatory strategies and hands-on support.
- Quality Assurance (QA): maintaining compliance and high-quality standards across development and operations.
- IT and Data Storage: safeguarding critical development data with secure, reliable infrastructure.
With this acquisition, we are extending our support further along the development pathway. By adding DGr Pharma’s consultancy and regulatory expertise to our integrated offering, we strengthen our ability to guide clients seamlessly from early discovery through development, registration, and manufacturing.
This partnership reflects our ongoing commitment to building a truly end-to-end service model. Together with DGr Pharma, we will be able to offer even greater flexibility, scientific depth, and speed, helping our clients bring high-quality medicines to patients worldwide.
We look forward to working closely with our new colleagues and building the next chapter of Symeres together.
Latest news

What are the top drug development trends for 2026? Exemplify CSO Paul O’Shea discusses what to watch

Symeres appoints Jurgen Berendsen as Chief Financial Officer

Symeres earns first SBTi approval as part of long-term sustainability journey

Symeres appoints Gabriella Gentile as Chief Operating Officer to drive transatlantic growth

Symeres and Yoneda Labs use AI to optimize cross-coupling reactions

New production lines D1 and D2 at our Prague site

New NMR technology – meet “Maggie”
Symeres announces leadership evolution

Official opening of new Oncolines home, the Marie Curie Building at the Pivot Park in Oss, the Netherlands

New equipment at Exemplify
New 100 L vessels in Groningen

Organix: New facility

Innovative macrocycles as powerful Mcl-1 inhibitors

Oncolines unveils new state-of-the-art laboratories

Peter Molenveld assumes role as secretary of KNCV (Royal Netherlands Chemical Society)

Chiral chemistry: in the DNA of Symeres
π-facial selectivity in the Diels–Alder reaction of glucosamine-based chiral furans and maleimides

Symeres acquires Oncolines, further strengthening its drug discovery and biology capabilities

Symeres acquires Exemplify BioPharma, further strengthening its strategic foothold in the US
Expansion of solid-state center of excellence

Symeres acquires Massachusetts-based Organix Inc., adding lipids expertise and creating a strategic foothold in the US market
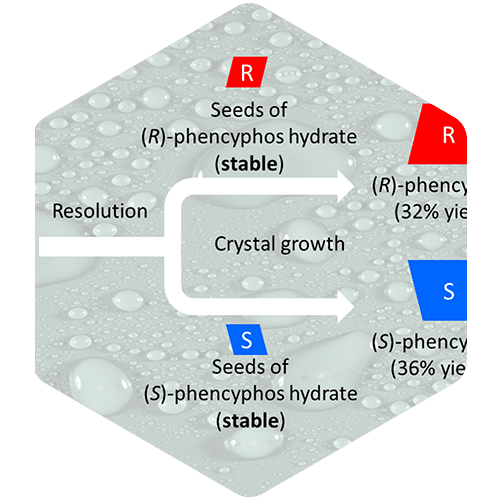
Expanding the toolbox: Resolving racemates

Symeres joins forces with Keensight Capital

Speak with our experts
Let’s discuss how Symeres can support the discovery and development of your next breakthrough