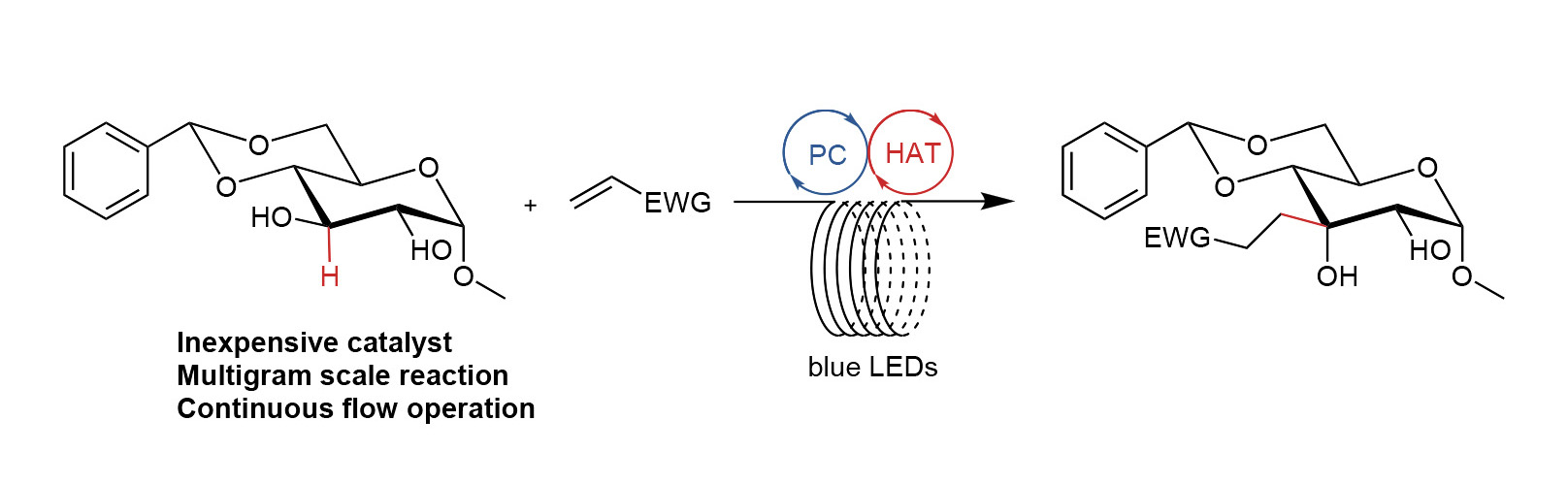
Site-specific alkylations extend the carbon framework of naturally occurring carbohydrates. Often, the methods to synthesize these compounds are laborious. For his PhD research, carried out at Symeres, Marc Mouthaan investigated a continuous-flow method, which allowed him to scale up the synthesis of carbohydrate scaffold precursors. The results have now been published in an article in Synthesis, which can be found here.
“My name is Marc Mouthaan, and I am a PhD student under the supervision of Prof. Adri Minnaard from the Stratingh Institute’s Chemical Biology section at the University of Groningen. Within Symeres, I am part of the group of Kees Pouwer. In my research, I convert a natural feedstock into an innovative scaffold, which can be used for library synthesis or as a building block.
The article describes how a glucose derivative can be alkylated site specifically with a variety of substrates in a photoflow setup, which, to the best of the authors’ knowledge, has not been done before. The photoflow setup allows the synthesis of multigram quantities of the products, which is sufficient for their use as building blocks for a variety of research routes.
“In the article, we also describe successful alkylation with a styrene and vinylpyridine,” says Marc. “Although the yields are moderate, this is the first time that such a coupling has been reported, and we are confident that, with a little more research, this reaction can be optimized.”
Working with photoflow equipment does have its technical challenges. “It took me a while to figure out how to prevent the tubing from clogging up under these specific reaction conditions, but, in the end, I had a robust setup to carry out the synthesis, and I could reliably reproduce the results.”
Clearly, this is just the first step of the research. Marc’s next investigations will focus on transforming these alkylated sugars into novel valuable scaffolds, which Symeres can use in its compound libraries.