Symeres has quite a legacy when it comes to university collaborations. For many years, we’ve been encouraging early research and bringing in students to learn the tricks of the trade. Many of these students are now part of Symeres’ much valued workforce. With their genuine passion for drug discovery and development, they expand our toolboxes in all kinds of fields.
Can you introduce yourself? What is your role within Symeres?
My name is Michel Leeman. I am 43 and I am a project leader in the group of Bas Dros. Last September, I celebrated my 20-year anniversary with the company. Before I became a project leader, I was a senior scientist specialized in the resolution of racemates by crystallization.
What is the project about?
I did my PhD research while working at Symeres. We had been working on the large-scale resolution of phencyphos. Phencyphos is a great resolving agent but, ironically, difficult to resolve itself.
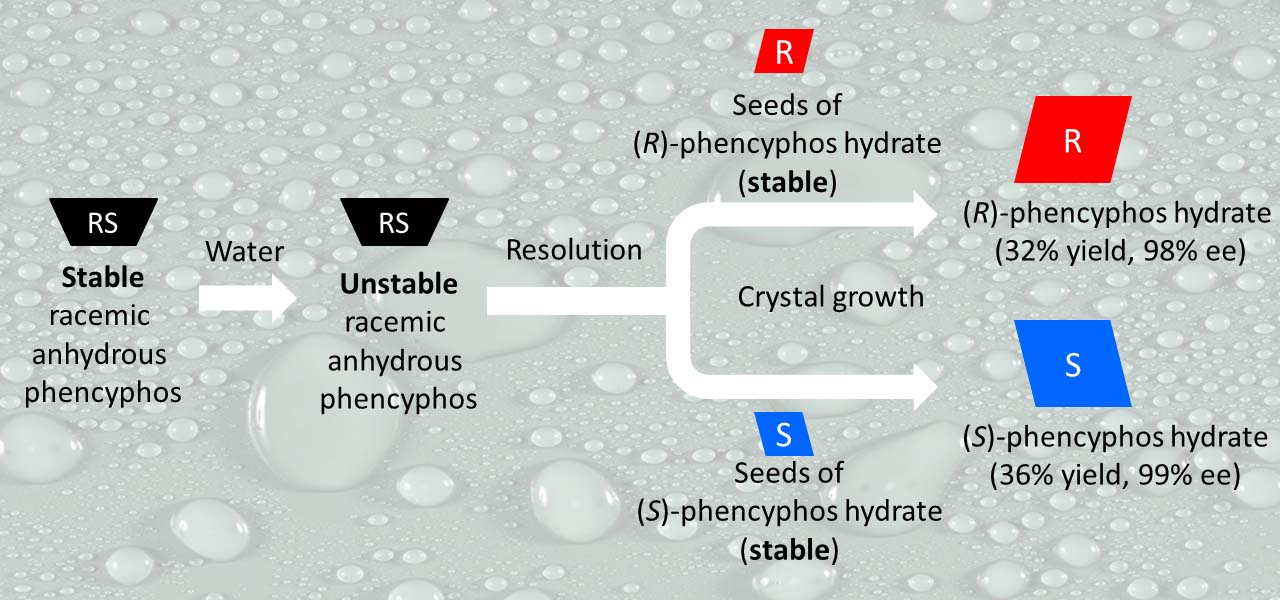
Anhydrous phencyphos forms a racemic compound, a crystal form in which both enantiomers are present in a 1:1 ratio. However, if enough water is present in the solvent used, a monohydrate crystal becomes the most stable (and thus, least soluble) crystal form. Unlike the anhydrous material, this hydrate forms a so-called conglomerate, a crystal form where each crystal is enantiomerically pure.
When enough water is present in the solvent, the racemic anhydrate slowly dissolves, and the solution becomes supersaturated in the monohydrate. By seeding this solution with both enantiomers, each in a separate compartment, the racemate is resolved into the pure enantiomers. A setup consisting of three stacked Soxhlet filters was built, containing the racemate, seeds of the (R)-enantiomer, and seeds of the (S)-enantiomer, respectively. A mixture of methanol and water was pumped through this setup. After a few days, the racemate had nearly fully dissolved. The seeding crystals had grown considerably and optically pure phencyphos hydrate was obtained, with a combined yield of 68%.
Who was involved in the work?
As the work was performed during my PhD research, the team working on this project was just my academic mentor, Professor Kellogg, and me. I recall that, at the time, I had this wild idea which might just work. Professor Kellogg was convinced too, and I started to build the setup from parts that were available in the lab. I still enjoy discussing chirality with Professor Kellogg, who is now a scientific advisor for Symeres.
What challenges did you overcome?
Before we could start the experiment, we needed to have data on the solubility of the anhydrous racemate and the monohydrate enantiomers. Earlier, we had performed a so-called preferential crystallization on phencyphos monohydrate (preferential crystallization is a cyclic method to resolve a racemate without the use of a resolving agent by alternatingly crystallizing the enantiomers). With this methodology, we isolated nearly 40 kg of each enantiomer.
However, setting up the preferential crystallization of phencyphos monohydrate required profound knowledge of the crystallization behavior of the monohydrate. We made ternary phase diagrams in different solvent systems and determined the metastable zone widths. This information was very helpful in setting up the resolution with the Soxhlet filters.
What achievement are you most proud of?
When you build a new system from scratch, usually you need to do some tweaking to get the experiment working. However, in this case, the resolution worked flawlessly on the first try. Starting from completely racemic material, we obtained the optically pure enantiomers in good yield in a completely hands-off method.
What impact do you think the results had?
When resolving racemates, there is no single method that gives the best result every time with respect to efficiency and costs. In this publication, we show that a metastable crystal form can be used to isolate an otherwise difficult-to-resolve compound in an easy and hands-off method. Although this may not be a general method to resolve all racemates, it is another useful tool in our toolbox. The publication is also a message to other chemists to study the behavior of a compound and to use this knowledge to their advantage.
You can find the full publication here (Crystals 2021, 11(10), 1225).