The Symeres SymeGold compound library contains a high number of innovative sp3-rich scaffolds and has recently become available to external parties for high-throughput screening. It is continuously updated with novel and high-quality scaffolds.
Together with the research group of Prof. Minnaard at the University of Groningen, Symeres is exploring opportunities to access novel chemical space by using carbohydrates as sustainable chiral starting materials for library synthesis. In 2021, we published a dehydration method to prepare a chiral furanic building block from the carbohydrate N-acetyl glucosamine. In our most recent publication, we show how this type of synthon can be used to access stereochemically dense scaffolds via a Diels–Alder cycloaddition (figure 1).
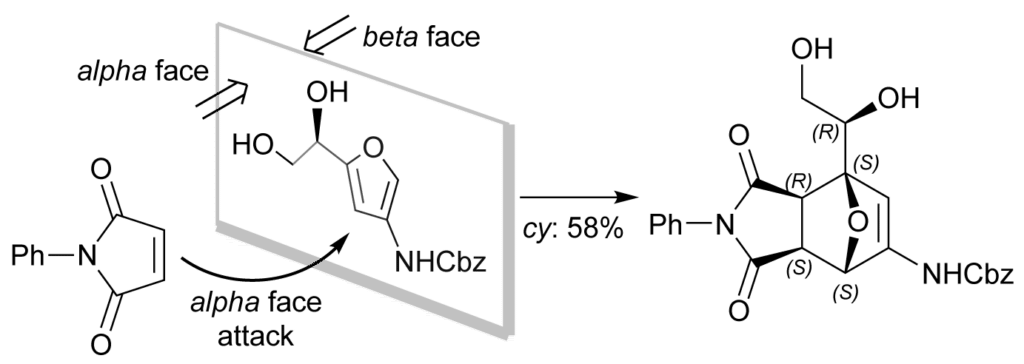
Due to their substitution pattern, most carbohydrate-based furans typically have opposite π-facial planes, and therefore, the Diels–Alder cycloaddition yields racemates. Glucosamine-based furans are unique, in the sense that dehydration under the right conditions affords optically active furans in high enantiomeric excess. This feature allowed us to induce face selectivity and prepare enantiopure amido oxanorbornene scaffolds with five chiral centers.
Can you introduce yourself? What is your role within Symeres?
My name is Kees van der Loo. I’m working to obtain my PhD degree at Symeres, in collaboration with the University of Groningen. In my research, I study the use of biorenewable carbohydrates as a sustainable starting material for library synthesis.
What is the project about?
The goal of the project is to find innovative ways to access novel scaffolds that can be used to synthesize biologically relevant compound libraries. Dehydration of glucosamine derivatives affords enantiopure chiral furanic building blocks with a substitution pattern that is not easily accessible synthetically. This makes them interesting starting points for the synthesis of a new class of stereochemically dense library scaffolds with a high degree of sp3-hybridized carbons.
What is the role of Symeres within the project?
In this project, Symeres is the industry partner that provides the laboratory facilities and contributes to the research with expertise in synthetic chemistry and knowledge on library scaffold synthesis.
What is the team like?
The Diels–Alder study was started by Rutger Schim van der Loeff, an intern at the time and now employee at Symeres. After Rutger graduated, I continued the research, which ultimately led to the publication. At the end of the project, the team got a more international feel, as a collaboration was started with the University of Alcala (Spain) to assist with X-ray crystallography.
What challenges have you and your team overcome?
Several analytical and synthetic hurdles needed to be overcome. In a single reaction, two relatively simple molecules produce a mixture of four diastereomeric products with complex three-dimensional structures and four new chiral centers. Due to the structural complexity and similarity of the products, both analysis and purification had interesting challenges. Reaction monitoring by 1H NMR spectroscopy was hampered by signal overlap in the NMR spectrum. This was solved by a preanalysis Malaprade oxidation step to remove the interfering structural moiety, which made the diagnostic proton signals visible. Purification challenges were overcome by combining column chromatography with a postpurification aqueous wash to suppress degradation during isolation of the product. Ultimately, the product was synthesized on a multigram scale in nearly 60% yield and >99% diastereomeric excess.
What achievement are you proud of?
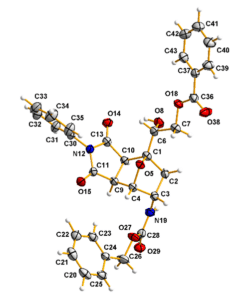
A scaffold was prepared with a total of five new chiral centers, and we needed to resolve the absolute stereoconfiguration. Based on a comprehensive NMR study, we narrowed the possibilities down to two plausible configurations. However, for conclusive proof, we had to resort to X-ray crystallography. Unfortunately, none of the intermediates were crystalline, and therefore, several derivatives were prepared. Eventually, we found a crystalline derivative, and we collaborated with the University of Alcala to obtain the crystal structure (figure 2), which brought the project to a very satisfying end.
Do you want to learn more about our synthetic capabilities or our Symegold library for HTS screening? Contact us!