High-throughput screening is a powerful tool to start drug-discovery programs. The success of this activity is highly dependent on the quality of the library that is screened. With this in mind, Symeres created the SymeGold library, which features compounds with a high degree of three-dimensionality. This library was originally created under the ELF initiative and is continuously updated by adding novel, high-quality scaffolds. Part of this initiative involved a collaboration with the Chemical Biology group of Prof. A. J. Minnaard at the University of Groningen. Two PhD positions were created to study the use of carbohydrates as sustainable starting materials for library synthesis.
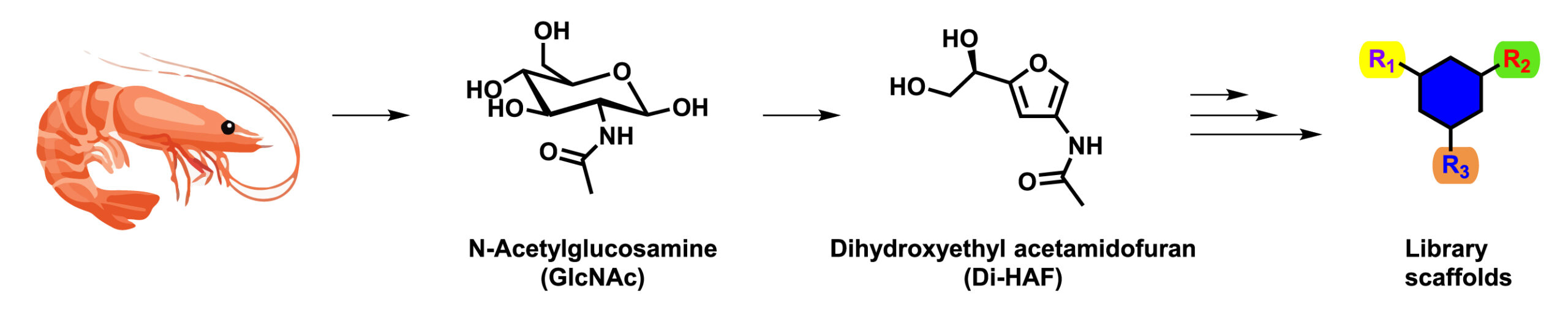
Kees van der Loo, a former research scientist at Symeres, is one of the PhD students on this project. His research focuses on using the biorenewable carbohydrate N-acetyl glucosamine (GlcNAc) as a starting material for scaffold synthesis. GlcNAc is the monomeric unit of chitin, the major component of the shells of crustaceans such as crabs, lobsters, and shrimps. Chitin is the second-most-abundant biopolymer found in nature, only surpassed by cellulose. Just like any other carbohydrate, GlcNAc is very polar and complex due to the large number of hydroxyl groups and chiral centers present. To make GlcNAc more suitable for scaffold synthesis, Kees decided to simplify the chemical structure of GlcNAc via dehydration, to afford furan dihydroxyethyl acetamidofuran (Di-HAF), a versatile molecule with multiple handles for further functionalization. Di-HAF opens the door to a new and interesting chemical space.
The synthesis of enantiopure Di-HAF had not been reported in the literature, and the first goal of the project was to develop such a process. Multiple challenges were overcome and, ultimately, a scalable process was designed that afforded Di-HAF in good yield (73%) and excellent enantiopurity (>99% ee). This novel route is now published in the journal Organic and Biomolecular Chemistry. Currently, Kees is investigating reactions to convert Di-HAF into interesting scaffolds by using rearrangement reactions and Diels–Alder cycloadditions.
Find the publication here.