This is a recap of the article “Development of potent Mcl-1 inhibitors: structural investigations on macrocycles originating from a DNA-encoded chemical library screen” published in the Journal of Medicinal Chemistry.
Myeloid cell leukemia 1 (Mcl-1) is a protein that plays a crucial role in the regulation of cell survival and cell death. It is part of a family of proteins called the Bcl-2 family, which are known for their involvement in controlling the life span of cells.
In our bodies, cells are constantly growing, dividing, and eventually dying in a controlled manner. This process is important for maintaining healthy tissues and getting rid of old or damaged cells. Mcl-1 helps to control this process by preventing cells from dying too early (apoptosis). However, in certain situations, such as in cancer, Mcl-1 can become overactive and help cancer cells survive and grow.
Scientists are developing drugs that specifically block Mcl-1 (inhibitors), with the aim of finding new treatments for some cancers in which Mcl-1 plays a key role in promoting cell survival.
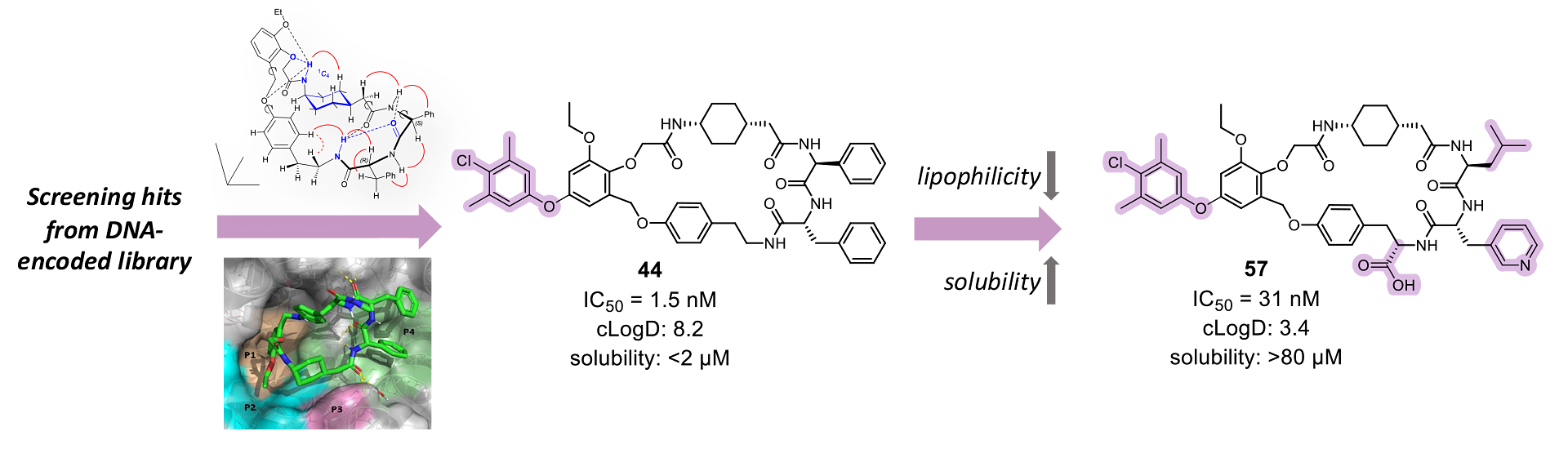
Several potent macrocyclic inhibitors have been developed during the last decade by companies like Amgen, AstraZeneca, and ABBV. All these compounds were synthesized through tedious processes starting from smaller noncyclic molecules. To shorten the pathway to the development of such drug candidates, Symeres in collaboration with X-Chem described the use of macrocyclic DNA-encoded chemical libraries for discovering such molecules.
The initial library was composed of 95 billion compounds covalently attached to a unique sequence of DNA, which permitted identification. This screening led to three macrocyclic hits with decent micromolar activities but poor physicochemical properties that had to be further optimized.
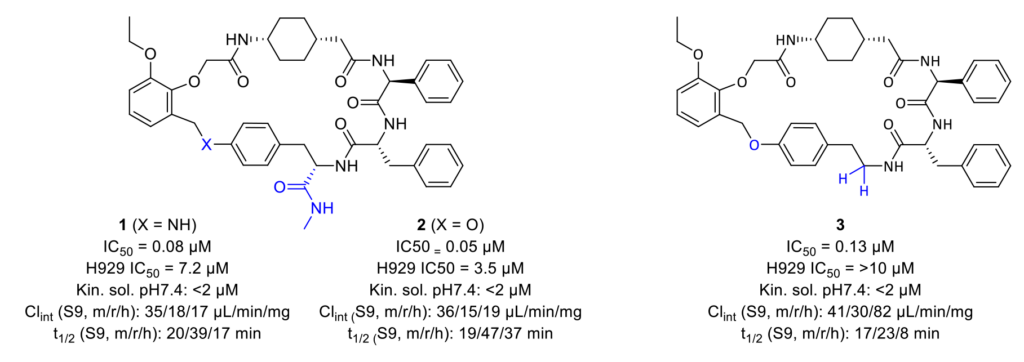
The binding mode of the macrocyclic compounds to Mcl-1 is crucial information for further optimization of the molecule. A combinatorial approach to investigate the binding of all parts of the molecules at the same time was used. Of a potential 733 target molecules, 444 were successfully synthesized and submitted to a Mcl-1-BAK TR-FRET binding assay. Forty-nine compounds demonstrated more than 50% inhibition at 10 µM, and finally, 15 compounds showed an IC50 value lower than 300 nM.
Some early structure–activity relationship (SAR) trends were immediately observed, and others were further investigated. The chirality of the amino acid fragments was studied, and the exchange of S-phenylglycine for the R enantiomer resulted in a dramatic loss of activity.
To get a better understanding of the SAR of the compounds synthesized, 2D NMR studies at variable temperatures and H/D exchange experiments in different solvents were performed. The structures of two isomeric macrocycles (active 3 and inactive 18 (compound numbers correspond to the J. Med. Chem. article)) were analyzed.
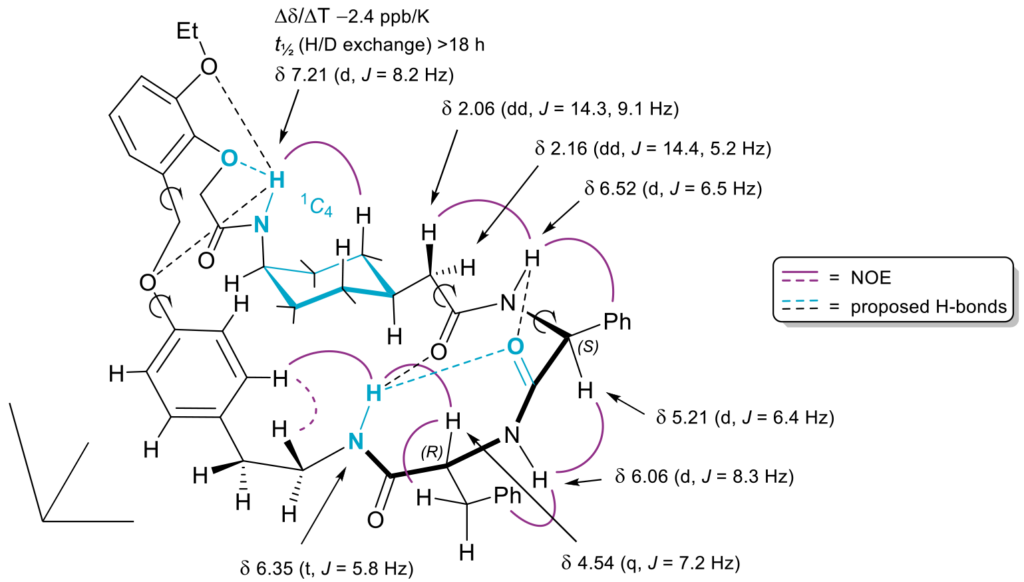
NMR studies showed that active compound 3 adopted a bent conformation in solution. The absolute configuration of the peptidic fragment is crucial since it affects the global conformation of the macrocycle. This conformation is essential for maintaining the binding affinity and the physicochemical properties of the molecule. Moreover, in the active conformation, the NH group of the phenylalanine is exposed to solvent and can be modified. However, the NH moiety in epimer 18 participates in an intramolecular hydrogen-bonding network and cannot be altered. The NMR conformational studies correlated perfectly with the SAR findings.
A protein–ligand cocrystal structure demonstrated the binding of the macrocycles to the BH3-helix binding groove typical of other Mcl-1 inhibitors. These molecules fill the most commonly found binding pockets, P1–P4, in the protein. However, an overlay study of the desired macrocycle with Fesik’s protein–ligand structure (molecule used as a model) showed that the macrocycle was not able to completely occupy the expanded P1 pocket (P1’ pocket), typical of the Mcl-1 protein, as other inhibitors typically do.
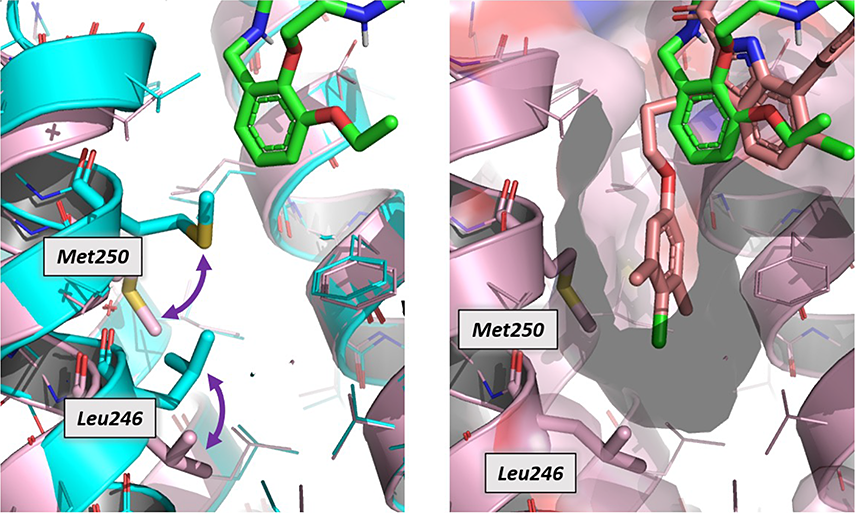
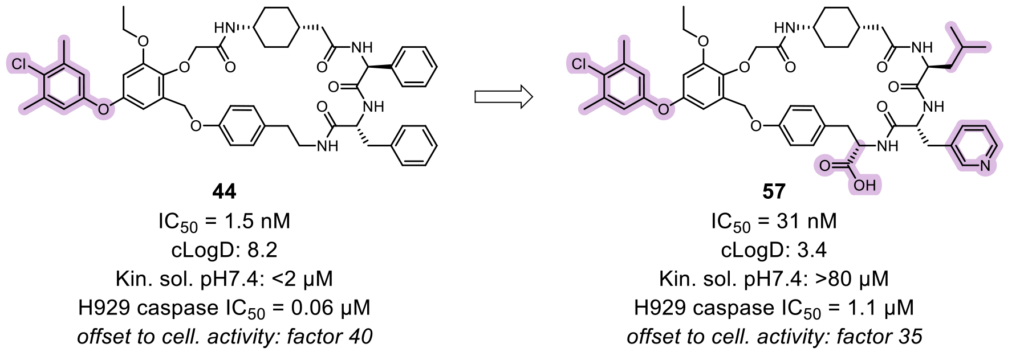
This structural knowledge-oriented lead optimization towards an increase of the affinity to the P1’ pocket. Two hundred new compounds were prepared with this aim. The introduction of biaryl alkynes improved the activity but, due to the rigid conformation, reduced the permeability. The introduction of aryl ethers improved the flexibility and permeability, thus achieving IC50 below 100 nM. Although properties such as potency, metabolic stability, and selectivity over BCl-xl were remarkable, the macrocyclic compounds showed poor solubility at physiological pH. This could be improved by increasing the polarity of the side chains. Lead candidates were finally selected based on their high activity and improved physicochemical properties of excellent aqueous solubility and moderate permeability.
This study successfully demonstrated how Symeres could support a fully integrated drug-discovery program from hit finding to clinical candidate selection.
Symeres has a strong track record in the validation and expansion of hits derived from different screening platforms, including high-throughput screening (HTS), fragment screening, AI-based virtual screening, and DNA-encoded libraries. The combination of high-quality integrated medicinal and parallel chemistry services can support and dramatically speed up drug-discovery programs.
If you want to know more about us, please contact us via our website. We will be delighted to assist you!
The article can be found at https://doi.org/10.1021/acs.jmedchem.3c02206