In the dynamic world of pharmaceuticals and biotechnology, finding a reliable long-term partner for drug discovery and development services can be challenging in terms of global location, timezone and logistics management. Symeres, with laboratories solely located in the USA and Europe, can significantly simplify your outsourcing strategy, offering scientific excellence, reliability, clear communication, and client-focus.

At Symeres, we pride ourselves on being large enough to progress multiple integrated projects, yet small enough to nurture personalized and lasting relationships with our clients, even where the needs are for small, single-service solutions. We understand the importance of every project we undertake, and our proactive communication style ensures that our clients are always informed and engaged at every step of the journey in direct interactions with the scientists they work with.
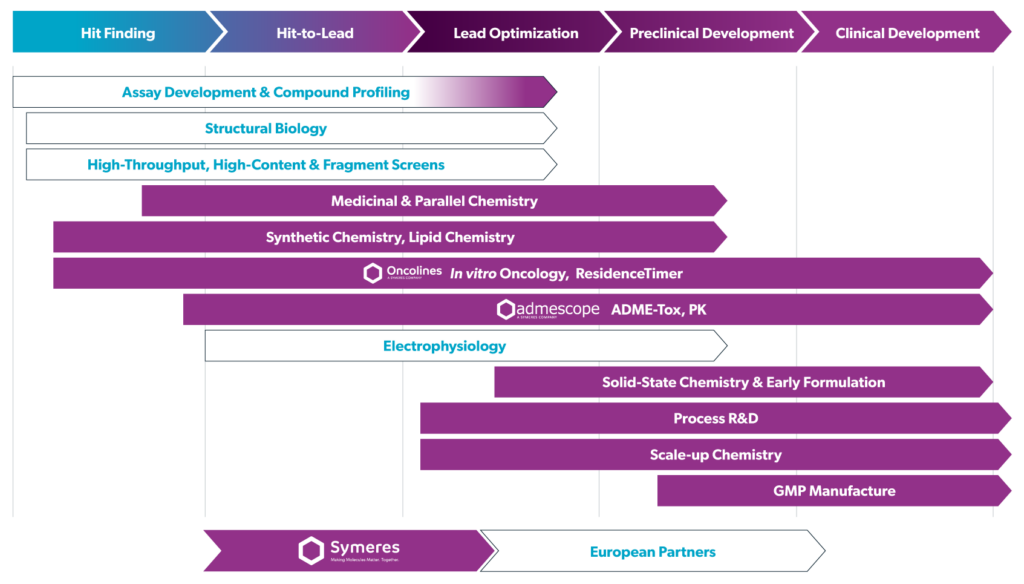
Our services span from the Discovery-stage, progressing from Hit-to-Lead through to Lead Optimization, with internal expertise in the design of molecules, via our computational chemistry and generating arrays via parallel chemistry to explore structure-activity and structure-property relationships. Complex synthetic chemistry, from mg to Kg scale, is in the DNA of Symeres and with several hundred highly experienced organic chemists located in our laboratories in the Netherlands, Boston MA and Princeton NJ, we are able to tackle projects however big or small. Compounds designed and made at Symeres can then be profiled both in our state of the art Admescope ADME-Tox and PK platform, and in robust in vitro biochemical and cellular assays, as well as our Oncolines® in vitro oncology assays and ResidenceTimer® SPR systems. Additional assays and platforms, including High Throughput Screening, DEL screens, structural biology and toxicology studies can be included in the project via our network of high-quality European partners, all project-managed for you by Symeres.
When it comes to drug development, Symeres is a one-stop destination for a wide array of services from early route-scouting and delivery of non-GMP batches for IND-enabling studies through process R&D to complete solid-state chemistry characterization, GMP drug substance manufacturing and now also formulation development and GMP drug product for Phase I and II clinical studies at our New Jersey site.
Diversification of outsourcing to mitigate project and study risk is a constant theme, and Symeres has already earned the trust of multiple large pharma and biotech companies to be their CRO partner in their ‘ASIA + 1 strategy’. These clients underscore our commitment to delivering exceptional results while adhering to the highest standards of creativity, quality and communication.
Trust Symeres to be your partner in your journey from concepts to transformative medicines. Contact us via the contact form, or directly via our VP Business Development, Dr. Bart DeCorte to discuss your needs with us.
